No Stone Unturned – Connor’s Pancreatic Cancer Journey
Thursday, September 19, 2024
We are grateful to be sharing the lived experience of Connor and Jen who have documented their personal journey with pancreatic cancer up until Connor’s passing in October 2024, to help other families cope with the challenges of this disease.
This blog article is from a series of content pieces written by these remarkable people.
Connor has approached his pancreatic cancer treatment with one goal in his mind – to be there for our boys. As a result, we have lived by a determined philosophy – no stone unturned. From the very beginning, Connor has been incredibly clear that he wants to do clinical trials and take an integrative approach to his treatment. First, we wanted to share our experience with genetic testing and finding and participating in a clinical trial.
Genetic results are in…
About three months after Connor’s diagnosis, we received the results of his genetic testing. We had been eagerly awaiting these results because they would inform whether there are targeted treatment options available. This information is incredibly helpful to assess what other treatments and/or clinical trials would be available to Connor.
There were two types of tests done:
- Blood test to assess for germ line mutations (genetic mutations that are inherited).
- Biopsy for full genome sequencing (genetic mutations that are somatic – mutations in the cancer cells in our body, not necessarily inherited).

Connor, Jen and their two sons in Cincinnati.
Our advice about the feasibility of genetic testing – don’t give up!
Pancreatic cancer can be sneaky in how it presents which can make it hard to get enough viable tissue to perform genetic testing. We knew this would be the case for Connor because he had multiple inconclusive biopsies of his pancreas throughout the diagnostic period. When Interventional Radiology was able to biopsy a node of metastases in Connor’s peritoneum, Connor was adamant that we get enough tissue and do as comprehensive a panel of genomic sequencing as possible with the tissue. Because of his persistence, we had thorough discussions with the oncologist about this and made sure to remind the surgeons prior to and after the procedure, ensuring that the tissue was picked up right away and sent to the lab. Connor was familiar with the delays and challenges in tissue sampling from his father’s cancer journey, otherwise we wouldn’t have been aware how much impact the patient and their family can have in moving this along and advocating. We are highlighting this for others going through this journey – don’t give up on getting some quality tissue and pushing for as much testing as possible, this can significantly expand treatment options!
For Connor, the genetic tests showed a few different mutations, the most promising of which included:
ATM mutation (germline) – this mutation did not show up in the tumour tissue, it was inherited from Connor’s dad, who also had cancer.
KRAS mutation (somatic) – This mutation is in 90%+ of pancreatic cancers, and by the time that Connor was diagnosed, it was well studied with little progress, but there was more promising research ongoing in 2022 and our oncologist felt at that time that the researchers may be on the cusp of something and she described this as the “holy grail of pancreatic cancer,” if a targeted treatment can be discovered. Note – she was right that they were onto something!
FGFR1 amplification (somatic) – This isn’t seen very often in pancreatic cancers, but it is in other cancers and there are drugs that target these types of mutations. Although further down the line of priorities, it still offered an option.
What the results meant for Connor…
Even before we considered a clinical trial, these genetic results helped to provide some information on how Connor may respond to chemotherapy, radiation as well as what existing targeted treatments could benefit him.
The ATM mutation is in a similar family to the BRCA 1 / 2 mutations and increases your risk of developing certain types of cancer when inherited. It also makes you more responsive to some treatments. For example, people with an ATM mutation often respond well to platinum based chemotherapy drugs, like Oxaliplatin. We know now that this was the case for Connor as he spent a year on Folfirinox, with good disease control and a partial response. Some people with the ATM mutation also respond well to the PARP Inhibitor Olaparib (Lynparza). This is an oral targeted treatment with far fewer side effects than chemo and no need to go into the hospital for systemic therapy. Connor spent three months on Olaparib before his disease progressed. Those with an ATM mutation also often respond well to radiation, which Connor has just started to receive for the management of bleeding and pain in August and September 2024.
(We will talk more about clinical trial options soon.)
What the results meant for our children…
The other benefit of getting the inherited genetic testing was to understand the impact for our children. In 2022, our children were 2 and 4 and because cancers associated with the ATM mutation are very rare in children, they have not undergone any testing. We are hopeful there will be a cure for cancer by the time they would need to be screened. Either way, we know early genetic testing and screening will be a priority when they are older.
Secondly, if both parents have an ATM mutation the children are at a high risk for developing a disease called Ataxia-telangiectasia (A-T) syndrome which is a rare neurodegenerative syndrome. A-T causes movement issues in children, often starting in pre-school and increasing in severity over time. It also significantly increases the risk of certain childhood cancers. The genetic counsellor recommended that I get tested as well to see if our children were at risk for this syndrome. Fortunately, the testing showed that I did not have this mutation – although testing is never perfect, this gave us a lot of comfort.
The search for trials begins…
Following the result of the genetic testing, we began our search for a clinical trial. There were two parts to this: first, we reached out to PANCAN, a US pancreatic cancer organization, second, we reached out to US institutions known for their cancer research and care. With PANCAN, at no cost to the patient, a case manager is assigned who carries out a comprehensive search for trials specific to your genetics. We reached out to other organizations but found the quality of trials and responsiveness through PANCAN to be phenomenal. Each time we had a new question or update, they provided a new list of trials.
We also started our outreach to various US hospitals in mid-2022 in an effort to get access to promising clinical trials and opinions from a variety of institutions. We didn’t wait until Connor started progressing on the first (or second) line treatment to start this outreach because we knew how quickly things could progress with this cancer and likewise how long it would take to find the right trial and get approved for it (they do a variety of testing to make sure you qualify for their clinical trial). We were not doing this outreach because we questioned the diagnosis or treatment approach at our cancer centre – we knew our oncologist was the best of the best and that she’s made the absolute right decisions along the way. The outreach was because of the speed of the science (it was changing quickly and we didn’t want to fall behind) and the access to clinical trials – which is unfortunately much easier in the US than in Canada.
An example of the speed of the science
When he was diagnosed, the most promising clinical trials were focused on his ATM mutation. By 2023, there were significant advancements in targeting KRAS and that became the priority trial area for Connor. Also, by 2023, a new target called MTAP deletion was established – this was so new that it didn’t even show up on the targetable section of my genetics report from the year prior.
Our advice about genetic testing reports
With respect to the science moving quickly, the other thing that can change is what mutations are considered oncogenic. As scientists discover more mutations, there may be potential targets that weren’t even known at the time your report was produced. It’s always good to check in and see if anything new has been identified and should be reported. For example, in 2023, an oncologist with a questioning mindset was reviewing Connor’s records and asked if he had an MTAP deletion. It turns out he did, it just wasn’t something that showed up in the two page report back in 2022 but by 2023 there were trials targeting this, some of which seemed pretty promising.
By the summer of 2023 there were some very promising clinical trials targeting KRAS – a mutation commonly seen in pancreatic cancer (> 90% of the time) as well as non-small cell lung cancer. For almost thirty years, this mutation was thought to be undruggable, meaning they couldn’t find a treatment that could get at it. These are early phase (phase 1 means first in humans), and they are focused on figuring out the right dose (the highest dose that can be given without having unmanageable side effects).
One of these trials seemed incredibly promising both to our oncologist and from our own research. The phase 1 trial for RMC 6236, a PAN KRAS inhibitor, was new and in high demand. Trials for this drug were only available at approximately 10 medical institutions in the summer of 2023, all of which were in the US. We took the approach of contacting most of the institutions to get on the waitlists – some would add Connor to the waitlist prior to an appointment, others with a virtual appointment and some required in person visits. We also realized most would put Connor on the wait list BEFORE he progressed on Folfirinox. Had we known, we would have started our outreach earlier.
Our advice about when to look into clinical trials
We were fortunate to have done a lot of research and asked about clinical trials at every oncology appointment, or at least included them on our email to the oncologist in advance of the appointment so we had had extensive discussions about which ones were most promising. We are glad we did this because when Connor’s scans showed that he was progressing on Folfirinox in July 2023 we had so little time to change course and the promising trials were all wait-listed. Thankfully we had made connections and acted fast to meet with the US institutions hosting the trials. We recommend starting this process – of what drugs are most promising and what institutions have the trials – while the chemo or more traditional treatment is working and while there is time to plan and do the research.
RMC 6236 in Cincinnati
On September 12, 2023, Connor got a call from Christ Hospital in Cincinnati that a spot had become available on the RMC-6236 trial. At this point, Connor’s treatment history looked like this:
• Folfirinox (Standard of Care – 1st Line) – May 2022 – January 2023 (16 rounds)
• Olaparib (Maintenance) – February 2023 – April 2023
• Folfirinox (Standard of Care – 1st Line) – May 2023 – July 2023 (6 rounds)
• Gemcitabine Nab Paclitaxel (Standard of Care – 2nd Line) (4 rounds, no progression, but got a spot on KRAS trial)
This seems like a lot of treatment, but our oncologist had made very wise decisions and Connor was able to go back to Folfirinox after Olaparib to get as much mileage as possible. We also spent a lot of time discussing whether to go on a clinical trial when Connor had not progressed yet on Gemcitabine Nab Paclitaxel – would it still work if he went back onto it later? After much thought and many opinions, we decided the clinical trial was just too good to say no to. We had also spent a lot of time doing everything we could to get on waitlists and were on about six. We expected the earliest a spot would come available was October, but more likely November/December, so we were ecstatic when he received the call.
In order to be a part of the clinical trial, many tests had to be done (EKG, ECG, CT scan, x-ray, bloodwork, physical, etc.). All of this had to be done on site at the trial location. In addition, you needed a three week wash out period from your last chemotherapy dose. Connor’s next chemo was quickly cancelled, and we began making plans to go down to Cincinnati for the screening.
Because all test related procedures/monitoring needs to take place at the trial site, Connor had to spend several days in Cincinnati, so we decided to make the most of a week away and brought the kids to Cincinnati.
After extensive screening, we got confirmation that the drug company that Connor was accepted into the trial. We were ecstatic! He spent a full day getting the trial drug followed by regular bloodwork and an EKG to monitor his heart’s response. He had to go back the next day for bloodwork. In the meantime, we were able to have a wonderful week in Cincinnati – with beautiful weather and a surprisingly vast array of activities for kids and families.
The trial medication is an oral drug. Initially, we thought we might need to stay in Cincinnati long-term but Connor was able to fly back and forth for the monitoring. Our oncologist in Toronto was incredibly supportive of this trial and continued to be his oncologist here in Toronto and follow along.
We were feeling very hopeful that Connor would benefit from the drug. While it’s not a cure, it was a step in the right direction and a reflection of significant advancements in the science even since he was diagnosed.
The outcome
The first set of scans were done at the five-week, two-day mark on the trial drug and the measurement of his primary tumour has shrunk by more than 50%. His oncologist in Cincinnati gave him a huge hug. Our oncologist in Toronto was overjoyed to see Connor and how well he looked, with the results of his scans and with the hope the trial drug brings to all her patients. Although this drug wasn’t expected to work forever, it gave us time and gives time for science to advance.
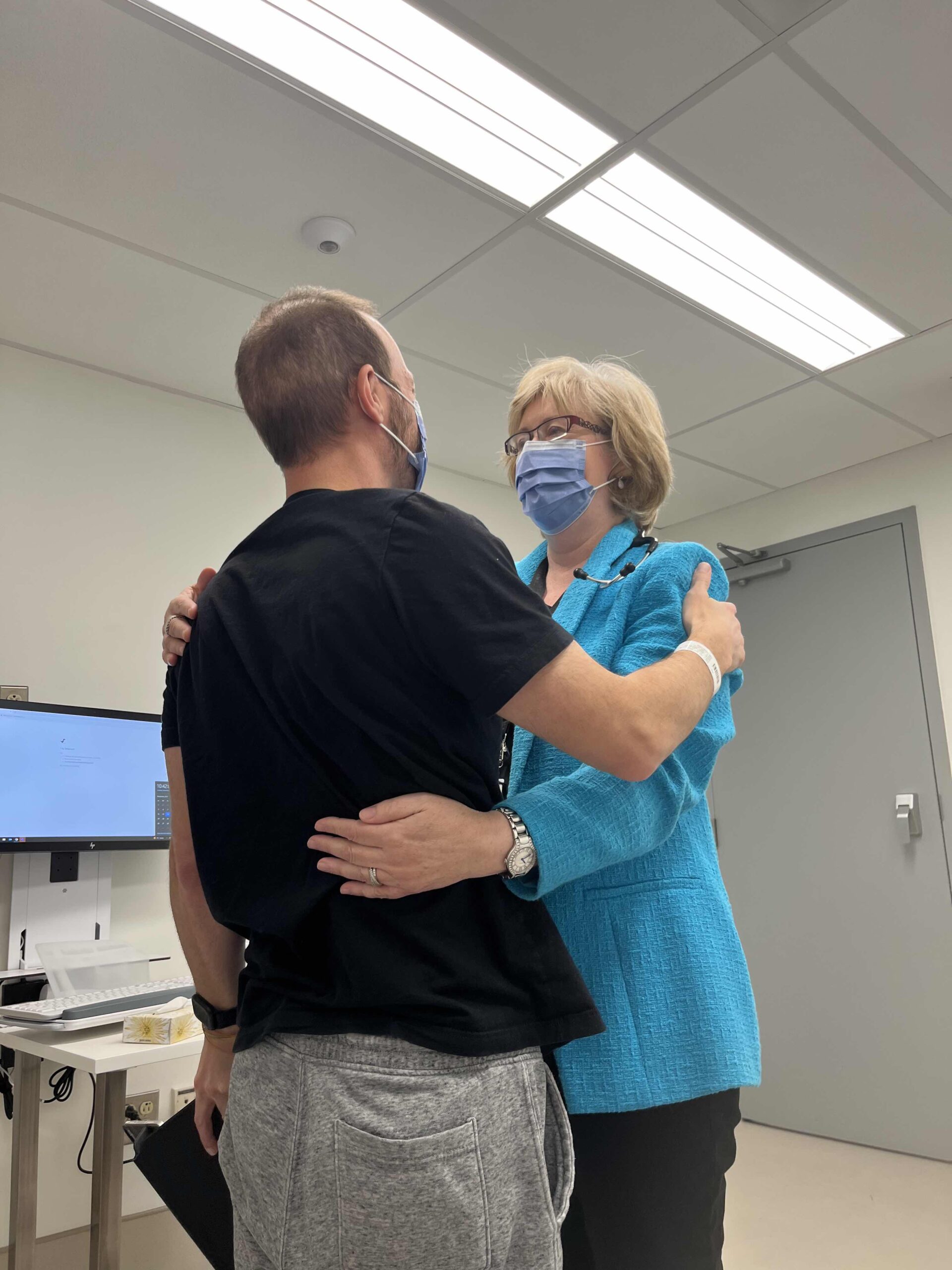
Connor and his oncologist, Dr. Jennifer Knox
We had several good months while Connor was on the trial drug – like doing F45 good, feeling the most like himself he had since the diagnosis. Well worth the effort for an incredibly memorable period of time.
Unfortunately, by March 2024, it came time to pivot treatments. With gradually increasing symptoms since January and rising tumour markers, Connor was concerned that the trial drug wasn’t working as well. Although his January scans were stable, his scans in March showed subtle changes. When combined with how he was feeling, the trial team concluded that the disease was progressing, and it was time to come off the trial drug. As ever, Connor was very in tune with his body and felt it was like the right decision.
We are incredibly grateful that we had the opportunity to participate in this trial. With every bit of hope we gained, we also know there are still patients out there who don’t get the opportunity to try some of the cutting-edge treatments (due to late diagnosis, ineffective chemo, lack of access, etc.). We hope to be able to take what we’ve learned and do our part to help support others.
Additionally, because of the smart decisions by his oncologists, Connor was able to go back to the Gemcitabine Nab Paclitaxel for a short period after the trial and to this day, his journey continues.
To carry on his legacy of selflessly helping others, we are proud to announce the Connor Page Fund for Improved Access to Clinical Trials. This Fund will allow his family to share, as he so aptly called it, Connor’s “Playbook” for navigating clinical trials and provide support to Canadians for years to come.
We graciously ask, if you are able, that you consider donating to this cause and join us in remembering this remarkable husband, father and patient advocate.

Recent Comments