NeoPancONE
Pancreatic cancer is a personal disease. We’ve learned this to be true right down to the molecular level.
Today we are using the information we’ve gained about the genomics of this disease to invest in a trailblazing clinical trial called NeoPancONE which aims to be a curative strategy for resectable pancreatic cancer.
Our past research investments have led us here.
From 2016 to 2018 we funded two clinical trials aimed at learning more about the genetic makeup of pancreatic cancer tumours to discover some of the many subtypes of this disease. This research lead to the discovery of five major subtypes of pancreatic cancer: Basal-like-A, Basal-like-B, Classical-A, Classical-B, and Hybrid, representing the most comprehensive analysis of the molecular subtypes of pancreatic cancer to date. These studies have furthered the possibility for patients to receive targeted treatments based on their subtype.
One of the most impactful things we learned from these trials was that the measurement of a potential biomarker called GATA6 is able to help separate tumour types into groups, enabling researchers to learn how patients would respond to different treatment paths based on their subtype. There have previously been no strategies for influencing treatment plans based on a person’s biomarkers. Until now – NeoPancONE.
What is NeoPancONE?
NeoPancONE is a phase II clinical trial that will provide chemotherapy to patients before and after surgery. Think of it like a surgery sandwich: chemo is given first to shrink pancreatic cancer tumours and then again after surgery to kill remaining cancer cells. This is an approach that has been taken in other cancers but not yet in pancreatic cancer. NeoPancONE is the first to use this treatment plan in consideration of a potential biomarker which will teach us more about how patients respond based on their subtype, helping them get the right treatment at the right time.
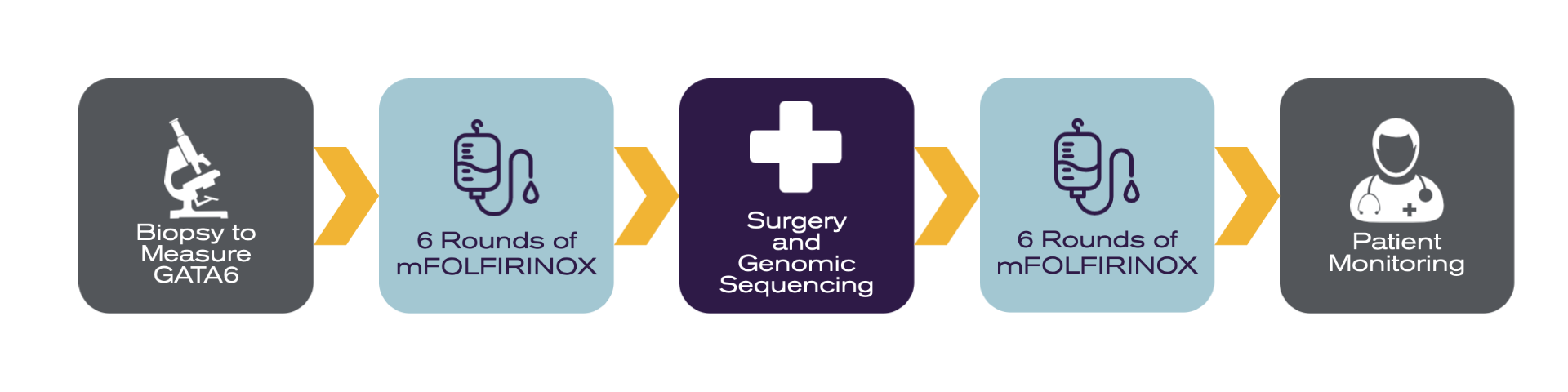
This is what the typical NeoPancONE patient journey looks like:
- Patients with operable pancreatic cancer will first receive a biopsy to measure their GATA6 expression.
- Eligible patients will then go on to receive six rounds of modified FOLFIRINOX (mFOLFIRINOX) chemotherapy to shrink cancer cells. (Receiving chemotherapy before surgery is referred to as “neoadjuvant chemotherapy.”)
- Patients then receive surgery to remove their pancreatic cancer tumour, which will be submitted for genomic testing to learn further about the patient’s disease subtype.
- Once recovered from surgery, patients will again receive six rounds of modified FOLFIRINOX to kill any remaining cancer cells. (Receiving chemotherapy after surgery is referred to as “adjuvant therapy.”)
- Once their chemotherapy treatment is complete, patients will be monitored for reoccurence.
By studying this innovative approach through a research-based clinical trial that includes the GATA6 gene, this study has the potential to offer eligible patients a treatment that leads to disease-free survival.
Why is NeoPancONE Important?
Neoadjuvant therapy has been used in other cancers, but it’s not yet standard of care for pancreatic. In fact, even the use of adjuvant chemotherapy is low in patients with this disease: typically, only 50% of pancreatic cancer patients receive chemotherapy after surgery. A further 20% of patients see their cancer progress before their chemo begins.
Recently there have been clinical trials that have included neoadjuvant therapy for pancreatic cancer, but never in combination with a potential biomarker study that can significantly impact and track the response of patients. That potential biomarker – GATA6 – is what makes this trial unlike any other.
By studying how patients respond to this trial, we will begin to understand which patients will benefit best from this treatment, learn about the aggressiveness of this cancer by subtype, and ultimately save more lives – for patients living with this disease right now and for those diagnosed in the future.
- The overall goal is to reduce the chances of disease recurrence, so more patients become survivors
- Will provide researchers an opportunity to learn about the aggressiveness of the cancer and how it reacts to treatment by subtype
- Will influence the standard of care so more doctors consider chemotherapy as a treatment that can lengthen – or save – a patient’s life
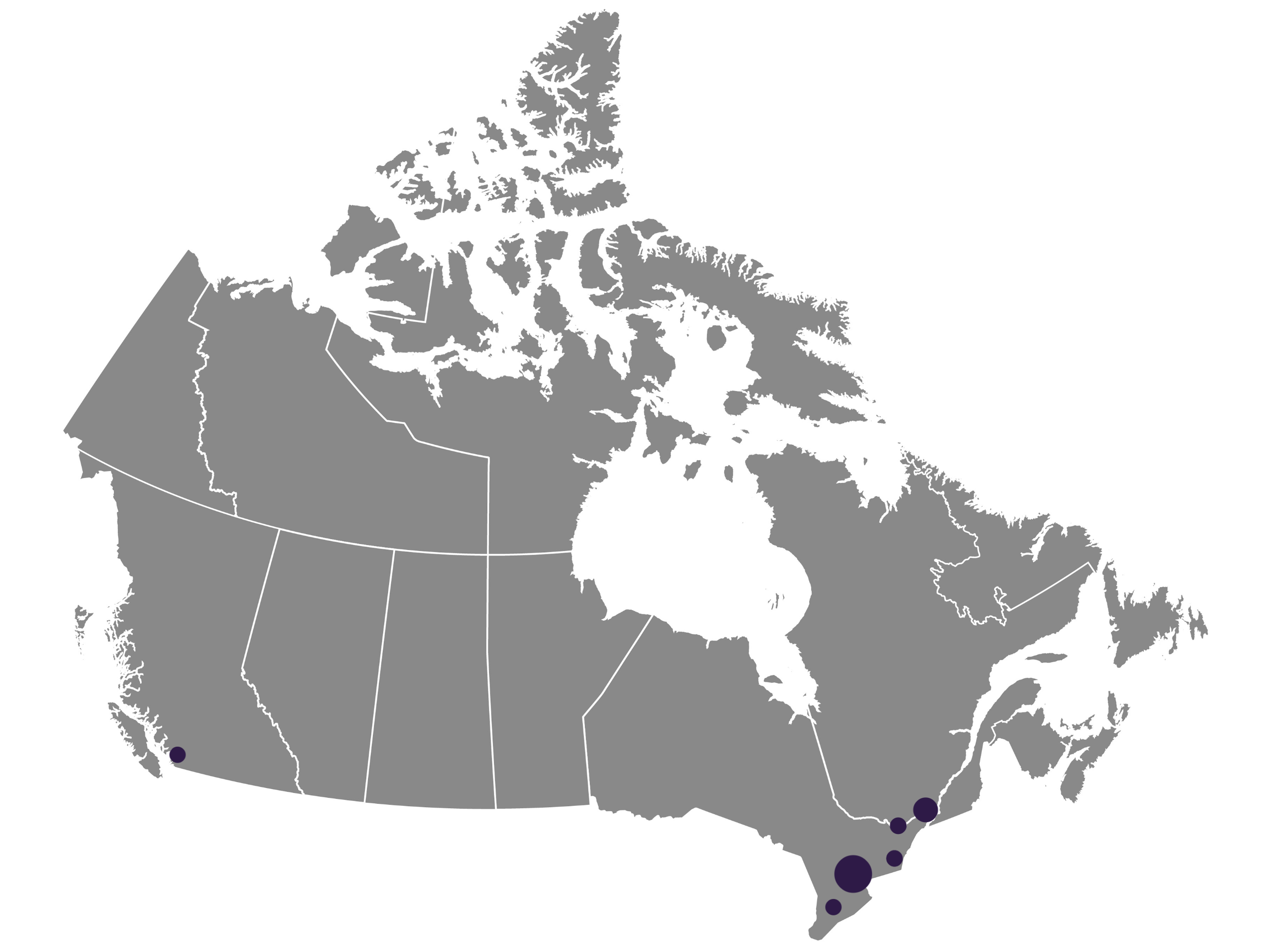
Participating Cancer Centres
- Princess Margaret Cancer Centre – Toronto, Ontario
- St. Joseph’s Health Centre – Toronto, Ontario
- St. Michael’s Hospital – Toronto, Ontario
- Sunnybrook Hospital Odette Cancer Centre – Toronto, Ontario
- Kingston General Hospital – Kingston, Ontario
- The Ottawa Hospital – Ottawa, Ontario
- Jewish General Hospital – Montreal, Quebec
- BC Cancer – Vancouver, British Columbia
- London Health Sciences Centre – London, Ontario
Further Resources
- PRESS RELEASE: Pancreatic Cancer Clinical Trial Launches During Pandemic With Aim to Change Standard of Care for Patients
- NeoPancONE Announcement: A Message from Michelle Capobianco
To learn more about the trial, please contact Anna Dodd, Research Liaison at anna.dodd@uhn.ca

316-4211 Yonge Street
Toronto, ON M2P 2A9
Toll Free: 1-888-726-2269
info@pancreaticcancercanada.ca
Charitable Registration Number 84870 1967 RR0001
